Salamander
- 52 Posts
- 88 Comments

 2·2 years ago
2·2 years agoThanks :D

 4·2 years ago
4·2 years agoThanks! Usually I see nice growth during spring and summer, but I find it difficult to get my more exotic plants through the winter. For this plant I want to make an extra effort. I can’t find much information about what conditions are optimal or even tolerated by this plant. So I’ll need some luck.
Cool! It’s like an antler coral forest.
I’ll go forage soon and make sure to bring you some nice pictures :-) I think it’s still a bit early for mushrooms in my area, though.
I’m not sure what is allowed here (no sidebar content?)
Ah, I’m sorry, that’s my fault 😅 I created a lot of communities at once and I never came back to properly create sidebars for all of them. My goal when I got started was to define the scope by creating communities about topics I find interesting… I will try to make some time to improve some sidebars. But, generally, I would say that if one is well-intentioned and not completely off-topic pretty much anything reasonable is allowed.
That said, if volunteers want to help moderate or take over a community, I’m always happy to hand over the reins!

 1·2 years ago
1·2 years agoThanks for the info! I’ll look over that and let it stew for a bit in my brain and will probably want to chat more. You can see where my general curiosity is pointed now, so the cordyceps information is perfect.
Always happy to chat! At this rate, by the next time we chat you will be the one explaining me things :D
After my first batch of mushrooms a couple months ago with no failures/contam, it gave me a huge confidence boost.
If failures do come, which is very very likely, don’t let that discourage you. Failures teach you important lessons.
I dove right into some of the more complicated topics shortly after. FWIW, I am compiling a spreadsheet that I intend to share that is a combination of LC recipes, substrate blends and various quantity calculators. Every YouTuber and website author has their own preferences, so I am basically going to average their data out and combine it with information I find in various research papers. (Testing is going to be the fun part though.)
Sounds super cool! When you do publish your results, please post about it or at least send me a message!
FFTFF is somewhat local to me in the Denver area and have been following that channel. It absolutely get my thumbs up.
Ohh. I’d absolutely go visit his stand at the farmer’s market if I was in the Denver area! But, yeah, I really like his methods, and he has lots of very interesting projects.

 2·2 years ago
2·2 years ago(Kind of a side topic) Since you mentioned mycelium age, I would guess that genetic degradation happens similar to cannabis. Granted, it can take dozens of generations for clones of clones to degrade in cannabis, it seems to me that mycelium might age faster? I dunno. That would make sense if it wasn’t for the prevalence of PE strains that have been around for years. (I am not a genetic scientist by any stretch.)
Oof, love this topic. Yes, mycelium ages and accumulates mutations. The extent to which it happens is species-specific, and I think the number of times that the cells have multiplied is more important than absolute “time”. So, usually you notice a culture aging as you sub-culture it, meaning that you will grow the fruit and clone the fruiting body. Cloning the fruiting body allows you to preserve a strain, but after a few cycles of doing this the strain begins to degenerate. So, that’s why one might want to keep a master of the strain under storage. Eventually, even that master may degrade, and that’s when you need to breed again from spores. One can grow fruits directly from a mixed population of spores, but the results can be varied. A strain is isolated when one wants more consistent results.
As I said, this is species-specific. I think Lion’s Mane and oyster mushrooms can do lots of sub-cultures. Psilocybin mushrooms can also handle a few before showing malformed fruits and losing their spores.
But if you really want to get into the cool stuff of strain degeneration… Cordyceps militaris. This is a mushroom that is becoming very popular and it is famous for its strain degeneration and the necessity to breed it - which is not so easy - this is a mushroom meant to grow as an insect parasite. If you are into the details of what is actually happening as this mushrooms is sub-cultured, I recommend this paper.
Here is a very cool image of what sub-cultures look like form that paper:
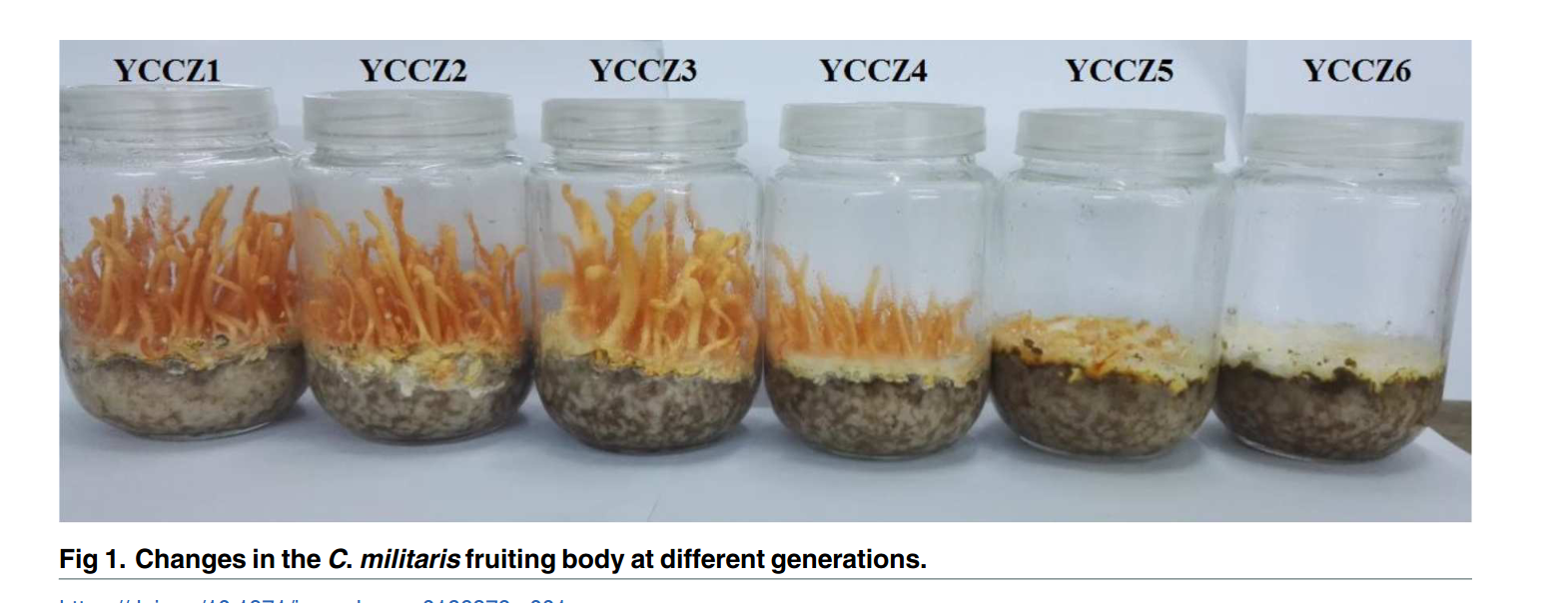
Working with this mushroom is a beautiful exercise of sourcing. Because of its popularity, many vendors are selling it now… But they will give you a strain that fruits like Z4/Z5. If you want a strain to fruit like first-generation fruiters you need someone who either collects spores from the wild, is a breeder, or knows how to source them and store them well. A youtuber I like watching (Fresh From The Farm Fungi) made a series of experiments trying to breed this fungus. He is very experienced, and you can see that even for him it’s quite a challenge: https://www.youtube.com/watch?v=NRm859-mjhM
But… yeah, this is probably more than you wanted to know about ageing in mycelium… Sometimes when I’m excited about a topic I talk a lot 😂

 4·2 years ago
4·2 years agoI thought about this and I decided that slants would be a better choice, but in practice I ended up using plates.
If you store a dish next to a slant for several months, you will notice that the dish eventually dries up. The slant remains hydrated for longer. Sometimes slants might also be more convenient to store or move around in a rack.
That said, as a small hobbyist I don’t really need to store strains for so long, and I prefer to refresh them often. So I just grow multiple plates at a time, do some agar to grain transfer, and stack the remaining clean plates in the fridge. This works fine, but I do refresh at least every 3 - 6 months.
I am more paranoid about liquid cultures and I don’t use them for long term storage. One excuse I can come up with is that if a contaminant reaches the liquid culture during long-term storage, it will mix throughout and it will contaminate the whole thing - whereas in the solid the contaminant might remain localized near the edges. I also have my crank theory about liquid cultures: I suspect that even if stored in the cold the mycelium will remain more active (and age faster) when suspended in a liquid than when resting on top of a solid medium. But this is pure speculation, I have not looked into the research, and maybe the mycelium rests just the same.

 2·2 years ago
2·2 years agoNice! Looking forward to some dirt talk!

 4·2 years ago
4·2 years agoI was surprised by their lack of smell, but I thought that the smell of the fresh flower might be subtle…
Eventually I figured out the disappointing fact that the scentless chamomile (Tripleurospermum inodorum) looks a lot like German Chamomile, and it is most likely what I have here 😭 So the quest continues… At least I know how to identify the real one now.

 1·2 years ago
1·2 years agoNoope, I’m pretty sure they are Tripleurospermum inodorum now 😭

 1·2 years ago
1·2 years agoAh! Yeah, I failed at reading comprehension here.
I don’t see the image you posted, but I looked up “D. carota dark central florets” and I found some images. Cool! I will pay attention to this next time I find them.

 2·2 years ago
2·2 years agoAh, actually, I responded thinking that you meant the /c/mycology community.
I’m not the creator/mod of the uncle_ben’s community, and the sidebar says “A community for the uncle ben’s mushroom growing technique.”, so I am actually not sure. I think it would be fine… Sorry!

 1·2 years ago
1·2 years agoSure, what’s wrong with bird seed tech?

 1·2 years ago
1·2 years agoThanks! I will look into those! Quite exciting :D
I have foraged and eaten some stinging nettle, but I didn’t know it was good for making tea!
Is there a trick to making elderflower tea? The crushed leaves of elder have a very characteristic strong smell that I don’t find so pleasant - will the flower tea taste like that?
The blackberry and wild strawberry teas are made from the fruit? Or can tea be brewed from the leaves?

 1·2 years ago
1·2 years agoHmmm, I vote for Matricaria chamomilla!
EDIT: I passed the other photos through PlantNet, and PlantNet agrees with me. I thought that the frame I chose for the post would be the best for identifying because it shows the leaves better. But now I have one suspicion: Most of the photos of Matricaria chamomilla that you can find by searching for images online feature the flowers, while photos of Chamaemelum fuscatum are taken with a similar framing to my input photo to showcase its dark stem. So maybe this bias in the framing of the photos present in the training set contributed to this miss-id. Just a thought.

 1·2 years ago
1·2 years agoCool observation about the hair! Thanks
I will study more details about the wild carrot and hemlock and I’ll inspect the plant better next time I walk by.

 51·2 years ago
51·2 years agoAll fungi are magical ;)
The mycology community is more general in scope. Psychedelic mushrooms are not excluded, but there can be so much specialized discussion in that sub-category that is worth it having a more specialized community.
There is also the first community [email protected] dedicated to growing, but it is not very active.

 2·2 years ago
2·2 years agoFrom Wikipedia:
The function of the central dark florets of D. carota has been subject to debate since Charles Darwin speculated that they are a vestigial trait.[14] It has been suggested that they have the adaptive function of mimicking insects, thus either discouraging herbivory,[15] or attracting pollinators[16] by indicating the presence of food or opportunities for mating
Alright - so it’s not only me who thinks that they look like spiky insects 😄
I wiped all my data from a supercomputer by trying to ‘cd’ into a folder but making a typo and then running
rm -rvf *from my home directory.At least they kept backups… The system administrators were probably amused about who gets access












AAAHHHH 😭 Such a relief. This was stressful.
The websites makes use of Contabo’s object storage service for storing and serving images. Earlier during the month, I got an e-mail saying that there would be a 12-hour downtime window due to a migration. Alright, no problem.
A bit before this users reported problems with uploads. I figured this was related to the migration.
After the “migration”, images still caused problems due to some “administrative rule” preventing uploads. I sent messages daily to Contabo. At first they were responding that “their engineers were looking into it, that this was related to the migration, and that they had no time estimate”. OK, I can be patient…
After a few days I lost some of my patience and pressed them more often, until they started sending me generic messages and eventually saying that “the problems were already fixed” and suggesting it may be some configuration error on my side.
This is where it got stressful, because my knowledge of the implementation of the images back-end is superficial. So… That meant I would need to study the details and either fix it myself or show proof that it was their fault.
These past few days I have tried to do some reading and tested configuration changes, but I am the kind of person that puts too much on their plate and I have been quite busy, so this has been an additional stress point.
Today, images suddenly started working (until it came down again due to some logs piling up). This tells me that it was not a configuration issue and it was with high probability something on Contabo’s side that they fixed today. Uploads are working as well now. Contabo support never shared any information about the specifics of what went wrong, so I don’t know.
This doesn’t mean that I don’t need to study the details, but at least now the pressure is lower. My plan now is to do some studying on how images are stored and served and once I have a good understanding I can migrate the instance to a dedicated server. I will also look into techniques for making a server more resilient when things do go wrong.